Abnormal blood flow and glucose metabolism in the putamen predicts Levodopa-induced dyskinesia in Parkinson’s disease
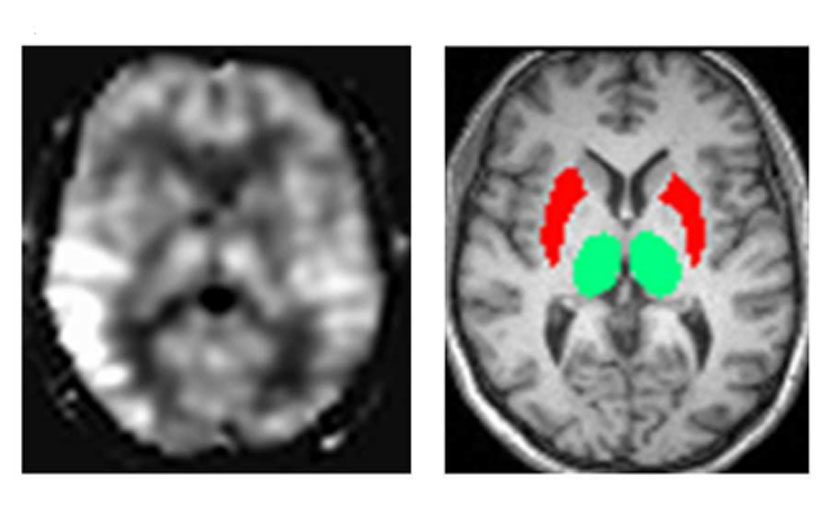
Levodopa is the therapeutic mainstay for the treatment of Parkinson’s disease (PD). It is used as a dopamine-replacement agent and is the most effective medication to control PD motor deficits arising from the degeneration of dopaminergic neurons and subsequent reduction of dopamine levels. Untreated PD is characterized by a hyper-metabolic brain, which is thought to reflect increased synaptic activity. While, neuroimaging studies show that dopaminergic therapy reduces and normalizes brain glucose metabolism (and therefore synaptic activity), this directly contrasts with the effects of treatment on changes in blood flow. Specifically, Levodopa (and dopamine) increases regional cerebral blood flow (CBF) via direct effects on the microvasculature (vasodilation) and induction of angiogenesis (the process of creating new blood vessels). Thus, dopaminergic treatment may be stereotyped by flow-metabolism dissociation, which has been implicated in the development of motor complications. Levodopa-induced dyskinesia (LID) is the most common complication of PD pharmacotherapy and manifests in more than 50% of PD patients 5-10 years after treatment is first initiated. Interestingly, patients with LID have a paradoxical reduction in dopamine levels at the synapse in the putamen region of the brain shortly after administration, whereas these levels are stable in patients without motor complications. This rapid putaminal dopamine turnover is thought to contribute to LID.
A study by Ko et al. from 2019 investigated flow-metabolism dissociation in the putamen and sought to identify a novel biomarker for LID. To examine glucose metabolism, brains were scanned using Positron Emission Tomography with fluorodeoxyglucose (FDG-PET). This work showed that FDG reuptake in the putamen was reduced with PD pharmacotherapy, suggesting normalized putaminal hypermetabolism. However, when perfusion MRI was employed, there were mixed effects on CBF. Together, these findings show a strong dissociation between blood flow and metabolic state, which was particularly notable in patients with LID. Past functional MRI (fMRI) studies have also implicated cortical regions of the brain in the development of LID, which led the group to examine dopaminergic modulation of cortico-striatal connectivity in LID. However, the results showed no differences in functional connectivity in LID versus non-LID patients between the putamen-primary motor cortex or between the putamen-supplementary motor area. The extent of flow-metabolism dissociation was quantified by comparing FDG-PET and perfusion MRI data to measure the putamen hyperperfusion/hyometabolism index (PHI) score. Ko et al. reported that the PHI score in patients with LID were above normal levels, suggesting PET + MRI (PHI) can be a useful and sensitive (80% sensitivity) biomarker for LID. The novelty of this work advances the search for a quantifiable LID biomarker that could predict the development of LID and improve the ability to monitor the progress of PD pharmacotherapy.
Reference: Aljuaid M, Booth S, Hobson DE, Borys A, Williams K, Katako A, Ryner L, Goertzen AL, Ko JH. Blood Flow and Glucose Metabolism Dissociation in the Putamen Is Predictive of Levodopa Induced Dyskinesia in Parkinson’s Disease Patients. Front Neurol. 2019 Nov 21;10:1217. doi: 10.3389/fneur.2019.01217. PMID: 31824400; PMCID: PMC6881455.
| Contributor | —Crystal Acosta |