Modulating the blood-brain barrier to treat brain tumours
Successful chemotherapy and targeted therapy of brain cancer has been extraordinarily difficult to achieve. This is in large part due to the blood-brain barrier (BBB), which is a specialized physical barrier formed by a network of endothelial and perivascular cells. The chief function of the BBB is to regulate paracellular permeability via junctional complexes between endothelial cells. These complexes restrict the passage of blood-borne particles into the brain and are therefore an important component of barrier permeability. Thus, the BBB is inherently protective against circulating toxins and pathogens, but it is this same property that poses a challenge to reaching therapeutically relevant drug concentrations in the brain.
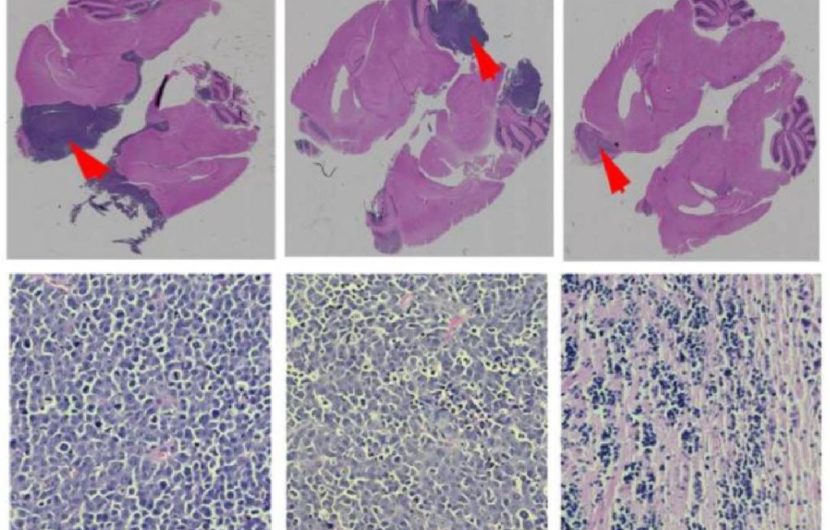
Cadherin is a junctional protein with a highly conserved His-Ala-Val (HAV) region that is required for the dimerization of cadherins. Peptides targeting HAV can prevent dimer formation and affect barrier permeability. In a study published in 2019 by Dr. Miller and group, the ability of HAV6 peptide to modulate the BBB and improve drug delivery was evaluated using a mouse brain tumor model. Previous work by Miller et al. identified Peroxiredoxin-1 (PRDX1) as a therapeutic target in Group-3 medullablastoma (MBL). MBL is the most common malignant childhood brain cancer, with the Group-3 subgroup being highly resistant to (radiation and drug) therapy and having the worst prognosis. PRDX1 prevents free radical-mediated oxidative stress, but is also believed to contribute to therapeutic resistance in cancer. Adenanthin (Ade), a naturally occurring compound derived from the Isodon adenantha herb, can inhibit the peroxidase activity of PRDX1 and has been shown to be effective in the treatment of cancers. Notably, Ade is BBB impermeable.
To evaluate the effect of HAV6 peptide on BBB permeability the brains of mice were imaged using MRI with gadolinium contrast agent. The results demonstrate systemic administration of HAV6 peptide caused a rapid and transient increase in barrier permeability in all brain regions assessed in vivo. Further, in silico predictions suggested Ade has low probability of crossing the BBB, which was consistent with in vivo findings. However, when Ade was co-administered with HAV6 peptide, brain concentrations of Ade were significantly increased. Moreover, toxic effects on cells was not observed with enhanced delivery of Ade using HAV6, suggesting concurrent administration of HAV6 with Ade can be implemented safely. To determine whether, HAV6 peptide improves tumor response to Ade and target PRDX1, Group-3 MDL mice were treated with Ade alone or in combination with HAV6. Co-administration of HAV6 and Ade improved tumor response and survival in mice versus those treated with Ade alone or with placebo. Further, molecular analysis showed increased DNA damage in tumor cells and increased tumor cell death. Together this study demonstrates that HAV6 peptide can enhance the delivery of Ade resulting in therapeutically relevant levels that can successfully target brain tumor cells. More importantly, this study validates the use of HAV6 for improving the delivery of BBB impermeable drugs to the brain.
Reference: Sajesh BV, On NH, Omar R, Alrushaid S, Kopec BM, Wang WG, Sun HD, Lillico R, Lakowski TM, Siahaan TJ, Davies NM, Puno PT, Vanan MI, Miller DW. Validation of Cadherin HAV6 Peptide in the Transient Modulation of the Blood-Brain Barrier for the Treatment of Brain Tumors. Pharma-ceutics. 2019 Sep 17;11(9):481. doi: 10.3390/pharmaceutics11090481.
PMID: 31533285; PMCID: PMC6781504.
| Contributor | —Crystal Acosta |